Nanion | Neuroscience 2021
Poster Presentations
Dr. Alison Obergrussberger (Nanion Technologies GmbH): Pharmacology of Transient receptor potential cation M8 (TRPM8) channels using different activation stimuli
海报展示Alison Obergrussberger博士(Nanion Technologies GmbH):使用不同激活刺激的瞬时受体电位阳离子M8(TRPM8)通道的药理学
Abstract:
摘要
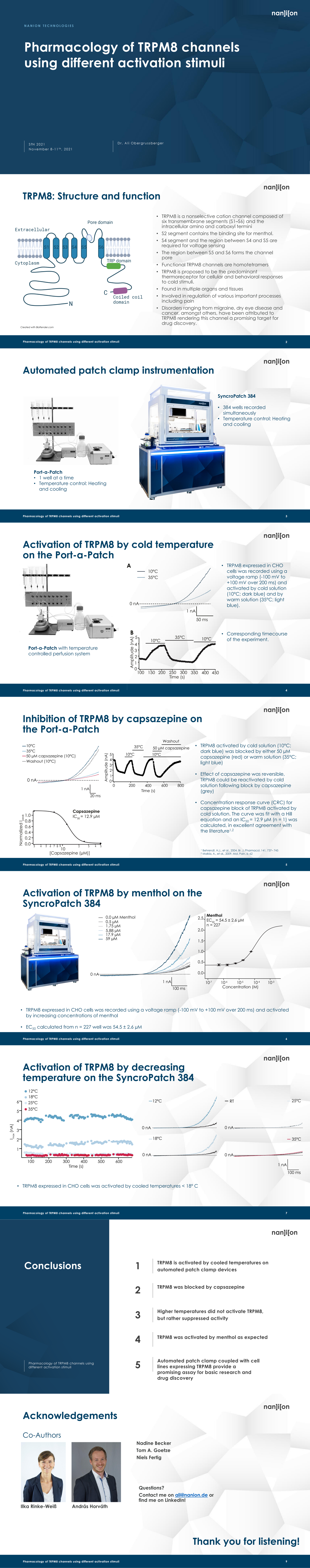
Transient Receptor Potential (TRP) channels are widely distributed throughout the mammalian central and peripheral nervous systems. The TRP channel subfamily M (melastatin) member 8 (TRPM8) is a nonselective cation channel, proposed to be the predominant thermoreceptor for cellular and behavioral responses to cold stimuli. TRPM8 channels can be found in multiple organs and tissues, and are involved in regulation of various important processes such as inflammatory reactions, immunomodulatory effects, pain, cell proliferation, migration and apoptosis, and vascular muscle tension. Disorders ranging from migraine, dry eye disease and cancer, amongst others, have been attributed to TRPM8 rendering this channel a promising target for drug discovery. Here, we studied the responses of TRPM8 expressed in CHO cells activated using different stimuli on automated patch clamp (APC) systems.
瞬时感受器电位(TRP)通道广泛分布于哺乳动物的*枢和外周神经系统。TRP通道亚家族M (melastatin)成员8 (TRPM8)是一种非选择性阳离子通道,被认为是冷刺激下细胞和行为反应的主要温度受体。TRPM8通道可在多个器官和组织中发现,参与调节炎症反应、免疫调节作用、疼痛、细胞增殖、迁移和凋亡、血管肌肉张力等多种重要过程。偏头痛、干眼症和癌症等疾病都被认为与TRPM8有关,TRPM8使该通道成为药物发现的一个很有希望的靶点。在这项研究中,我们研究了在自动膜片钳(APC)系统上使用不同刺激激活的CHO细胞中表达的TRPM8的反应。
TRPM8 is activated by chemical cooling agents including menthol and icilin or by temperatures lower than ∼26 °C. Using a high throughput APC device, TRPM8 was activated by increasing concentrations of menthol with an EC50 of approximately 20 µM. In addition, TRPM8 was activated when the system was cooled to 18°C and further activated at 12°C. A small, lower throughput APC device was also used to activate TRPM8 repetitively using solution at 10°C. This was subsequently blocked by increasing concentrations of capsazepine applied at 10°C with an IC50 of 12.9 µM. External solution warmed to 35°C also blocked the TRPM8-mediated current.
TRPM8被化学冷却剂激活,包括薄荷醇和icilin或温度低于26℃。使用高通量APC装置,TRPM8被激活,增加浓度的薄荷醇,EC50约20 m。此外,TRPM8在系统冷却到18℃时被激活,在12℃时进一步被激活。低通量APC装置也被用于在10℃溶液中重复激活TRPM8,随后在10℃时增加辣椒素浓度,IC50为12.9 m,从而阻断TRPM8介导的电流。外部溶液加热到35℃也阻断了TRPM8介导的电流。
To this end, TRPM8 could be reproducibly activated by different stimuli, and blocked by pharmacological agents or heated solution. Therefore, the use of TRPM8 expressed in cell lines coupled with high throughput APC provides a promising tool for basic research and may have important implications in drug development for compounds acting on TRPM8.
为此,TRPM8可被不同刺激重复激活,被药物或加热溶液阻断。因此,利用细胞系表达的TRPM8结合高通量APC为基础研究提供了一个有前途的工具,并可能对TRPM8作用的化合物的药物开发具有重要意
Rocco Zerlotti (Nanion Technologies GmbH): Solid supported membrane-based electrophysiology (SSME) electrophysiology meets SGLT1 and GAT1
Rocco Zerlotti (Nanion Technologies GmbH):固体支持膜基电生理学(SSME)电生理学满足SGLT1和GAT1
Abstract:
摘要
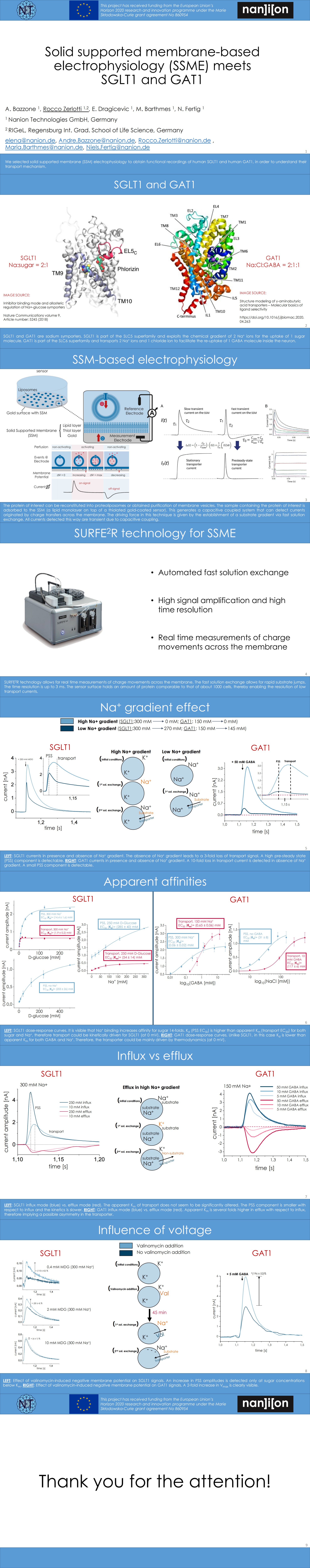
Transporter assays are often limited by the availability of labeled substrates and lack real-time data. Here, we developed functional assays to characterize the human Na+/Cl−/γ-aminobutyric acid (GABA) and Na+/glucose co-transporters - GAT1 and SGLT1 - using solid supported membrane-based electrophysiology (SSME). This approach overcomes several bottlenecks of other techniques and provides new insights into GAT1 and SGLT1 transport mechanisms.
转运蛋白测定常受标记底物可用性和缺乏实时数据的限制。在这里,我们开发了功能分析,利用固体支撑膜基电生理(SSME)来表征人类Na+/Cl /γ-氨基丁酸(GABA)和Na+/葡萄糖共转运体- GAT1和SGLT1。这种方法克服了其他技术的几个瓶颈,并为GAT1和SGLT1传输机制提供了新的见解。
In conventional electrophysiology voltage steps are used to trigger pre steady-state (PSS) and transport currents, which are commonly recorded in whole cells at a defined holding potential. Transport and PSS electrogenicity in SGLT1 and GAT1 triggered by voltage steps is postulated to be a result of transitions within the sugar and GABA-free carriers, e.g. the alternating access of the charged sodium binding sites within the empty carrier. In contrast, SSME utilizes membrane vesicles at 0 mV and the transport cycle is triggered by applying a substrate concentration gradient as the main driving force. Using SSME, we observed substrate-induced PSS currents, most likely representing conformational transitions within the substrate-loaded carrier, which are not observed with conventional electrophysiology. We examined the impact of different driving forces on influx, efflux, and PSS currents, focusing on sodium gradients and membrane voltage. We found that internal accumulation of sodium strongly reduces Vmax, rendering sodium release rate limiting at 0 mV. Application of membrane voltage only affected the apparent KM in SGLT1, but Vmax in GAT1. We also found that transport properties in GAT1 mediated influx and efflux modes are highly asymmetric, while SGLT1 has similar properties for influx and efflux.
在传统的电生理中,电压阶跃被用来触发前稳态(PSS)和传输电流,通常记录在一个确定的保持电位下的整个细胞。电压阶梯触发SGLT1和GAT1中的传输和PSS致电性,被认为是糖和无gaba载体内部过渡的结果,例如,空载体内的带电钠结合位点的交替进入。相比之下,SSME在0 mV时利用膜囊泡,并通过底物浓度梯度作为主要驱动力来触发转运循环。使用SSME,我们观察到衬底诱导的PSS电流,最有可能代表衬底负载的载流子内的构想转变,这是传统电生理观察不到的。我们研究了不同驱动力对内流、外流和PSS电流的影响,重*关注钠梯度和膜电压。我们发现,钠的内部积累大大降低了Vmax,使钠的释放速率限制在0 mV。膜电压的施用仅影响SGLT1的表观KM,而影响GAT1的表观Vmax。我们还发现,GAT1介导的内流和外排模式的转运特性高度不对称,而SGLT1具有类似的内流和外排特性。
致各位尊敬的客户:2021年元旦即将来临,根据政府部门的放假通知,结合我司的实际情况,现将我司2021年元旦
这一次的跨年,是2021年的期许,跟内心一样,想要平安健康就好。而让我们更加快乐的,有时候又是那一点点额外的
在这春意盎然、万物生长的时节凌云光与您开启2021年追光之旅DY站与您一起见证,光电行业的“新新”向荣!……
2021年第十四届深圳国际电池技术展览会 圆满落幕CIBF是ZG电池行业第之一个通过商标注册保护的
为积极推动表面分析应用技术的发展,促进表面分析技术与其它学科的融合,更好
2023年4月18日,2021-2022年度浙江大学药学院LOGAN奖学金颁奖典礼于浙江大学紫金港校区临水报
2020年11月6日,优普召开了“凝心聚力·策划未来”202
要点导读:2020财年第四季度●第四季度营业收入为14.8亿美元,同比增长8%;在所有地区和全部三大业务集团
11月24日,科学分析仪器领域最负盛名的权威期刊和在线平台Wiley Analytical Science (威利
由北京东方德菲仪器有限公司独资赞助,ZG化学会全国胶体与界面化学专业委员会设立的“东方胶化杯”全国胶体与界面